| Drug Name |
Enrofloxacin
|
| Synonyms |
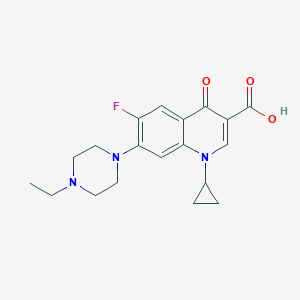
Enrofloxacin; 93106-60-6; Baytril; Enrofloxacine; CFPQ; Enrofloxacinum; Enrofloxacino; endrofloxicin; BAY VP 2674; Bay-Vp-2674; Enrofloxacine [French]; Enrofloxacinum [Latin]; Enrofloxacino [Spanish]; UNII-3DX3XEK1BN; N-Ethylciprofloxacin; Baytril (TN); ERFX; Enrofloxacin [USAN:BAN:INN]; 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; HSDB 6952; 1-Cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid; 3DX3XEK1BN; Enrofloxacin (USAN/INN)
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
359.4 |
|
| Logarithm of the Partition Coefficient (xlogp) |
-0.2 |
| Rotatable Bond Count (rotbonds) |
4 |
| Hydrogen Bond Donor Count (hbonddonor) |
1 |
| Hydrogen Bond Acceptor Count (hbondacc) |
7 |
| Chemical Identifiers |
- Formula
- C19H22FN3O3
- IUPAC Name
1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxoquinoline-3-carboxylic acid - Canonical SMILES
-
CCN1CCN(CC1)C2=C(C=C3C(=C2)N(C=C(C3=O)C(=O)O)C4CC4)F
- InChI
-
InChI=1S/C19H22FN3O3/c1-2-21-5-7-22(8-6-21)17-10-16-13(9-15(17)20)18(24)14(19(25)26)11-23(16)12-3-4-12/h9-12H,2-8H2,1H3,(H,25,26)
- InChIKey
-
SPFYMRJSYKOXGV-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 71188
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- VARIDT ID
- DR01565
|
|
|
|
|
|
|
|



